El sistema de trazabilidad ONELITE permite procesar lotes de medicamentos creando códigos unívocos, según las recomendaciones del estándar GS1, para cada unidad (en orden correlativo o aleatorio). Al mismo tiempo que crea una base de datos de fácil visualización, con acceso restringido según niveles de usuario bajo la seguridad que dicha información requiere de acuerdo con CFR 21 parte 11 (FDA).
El equipo de serialización ONELITE cuenta con impresoras InkJet de transferencia térmica, capaz de grabar dígitos alfanuméricos y códigos de barra unidimensionales y bidimensionales como los GS1 DataMatrix de aplicación principalmente farmacéutica.
Además cuenta con un sistema de visión artificial para realizar la verificación de la información impresa, mediante captura rápida de imágenes con iluminación integrada y lentes con herramientas de visión para inspección de códigos 2D y OCR (Reconocimiento Óptico de Caracteres). También es posible realizar una verificación cruzando los datos obtenidos, entre los textos humanamente legibles y códigos 2D para asegurar la integridad de datos.
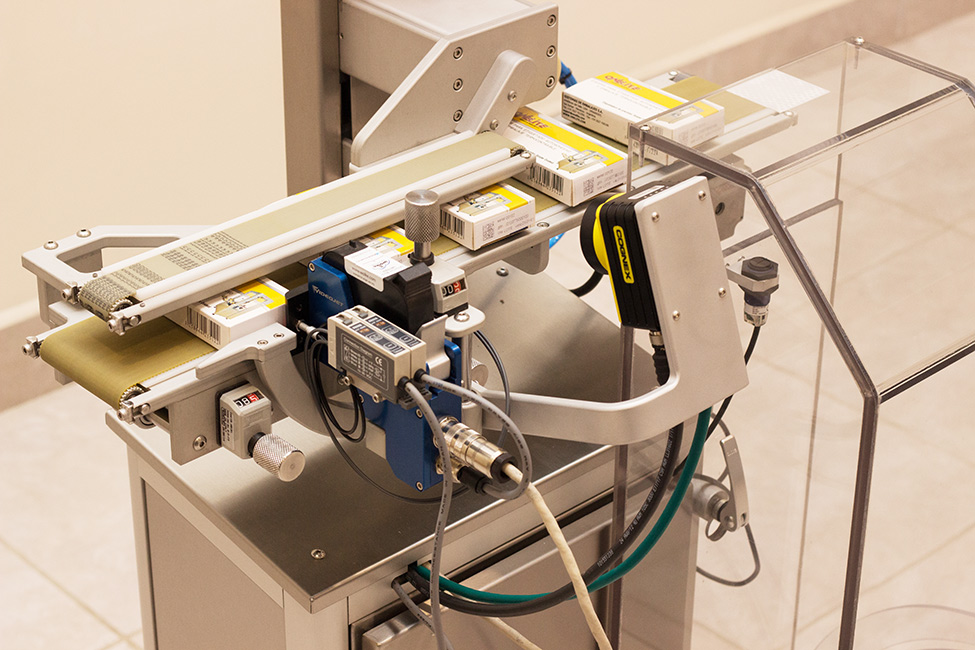
Mecánicamente el equipo cuenta con un transportador regulable para una gran variedad de estuches, asegurando un correcto desplazamiento libre de vibraciones en un amplio rango de velocidades. Conjuntamente cuenta con un sistema de expulsión neumática capaz de retirar de la línea cualquier estuche que contenga códigos incorrectos/ilegibles al cajón de descarte, siendo finalmente detectados por un sensor al ingreso del mismo.
La TPVA requiere un mínimo espacio para su ubicación e instalación y ofrece una fácil operatividad para su funcionamiento desde la pantalla táctil de 15”, coordinando los procesos de verificación, impresión y descarte, creando una base de datos para el rastreo de productos.
PhC Linha Pharma / Cométicos: TPVA
Módulo de serialización para trazabilidad de medicamentos TPVA
Equipo que permite la impresión y verificación de datos de trazabilidad en estuches de cartón, creando y asignándole un código seriado para cada estuche del lote. Además asegura que la misma sea legible por humanos de forma tal que permita la verificación por parte de los pacientes, en las condiciones normales de conservación del producto durante toda la vida útil.
Galeria de Detalhes
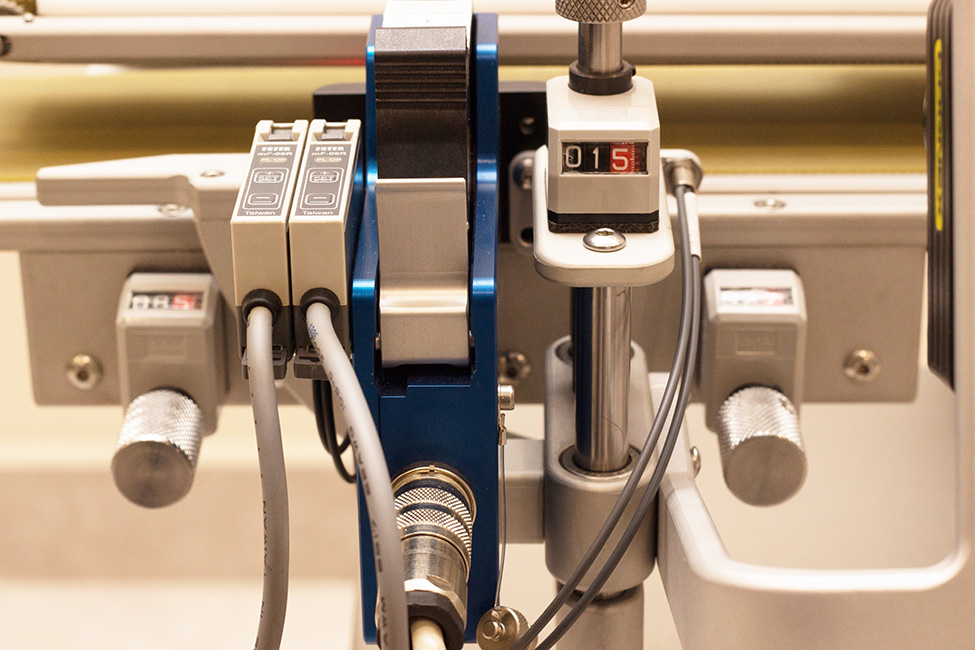
TPVA Detalhe
TPVA Detalhe
TPVA Detalhe
TPVA Detalhe
TPVA Detalhe
Características Destacadas
- Completa coordinación entre la impresión, la captura de imagen y el control de expulsión.
- Fácil cambio de formato (ajustes mecánicos y parámetros digitales).
- Control de calidad según lineamientos dados por GAMP5 (ISPE) y CFR 21 parte 11 (FDA).
- Ajuste de velocidad en modo continuo desde un solo parámetro en el panel táctil.
- Seguridad redundante de expulsión mediante la detección de ingreso de producto rechazado dentro del cajón de recogida.
- Generación/importación de seriales para procesar en el equipo.
- Visualización y descarga de reportes de producción detallados.
- Mínimo espacio requerido.
- Generación/restauración y descarga de backups del sistema.
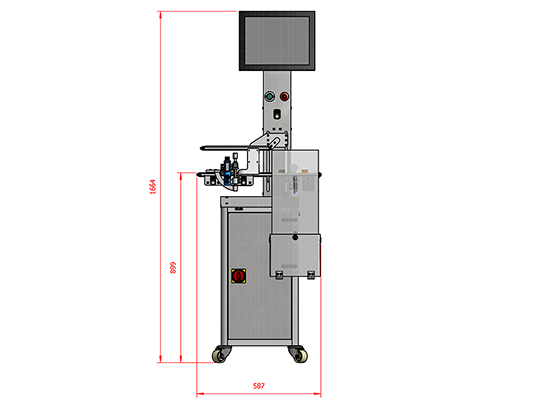
Dimensões Reais
Layout frontal
Layout Superior
Especificações Técnicas
<
| Dimensões (mm) - Comprimento x Largura x Altura | 587 x 600 x 1664 |
| Peso (kg) | 60 |
| Potência Instalada (KVA) | 1 |
| Nivel de ruído (dB (A)) | ≤ 70 |
| Production capacity (units/min) | 100-400 |
| Speed Range (meters/minute) * | 10 - 40 |
| Admissible carton sizes (mm) - Lenght x Width x Height | 30-120 x 15-85 x 80-180 |
| Admisible Labels (mm) - Lenght x Width | 25-50 x 15-40 |
| Printin Area (mm) - Height | 12,7 |
| Impressora | Wolke m600 OEM |
| Camera | IDS (Alemanha) UI525xLE-M |
| * Depending on the acquired version of the TPVA, the production can reach 100/200/300/400 units/minute maximum, for cartons which length is equal or lower than 80 mm. For longer cartons, maximum production may be affected. | |